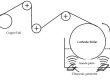
The charging and discharging process of lithium-ion batteries is realized by the intercalation and removal process of lithium ions in the layered electrode material, in which lithium ions will only cause changes in layer spacing, but will not cause other changes.
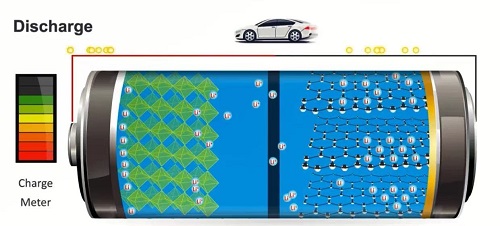
Charging and discharging lithium-ion batteries
So, the coulombic efficiency of lithium ion batteries is high, and it can also be considered as a more ideal reversible battery.
Charging
In the charging process, the external circuit charges the electric vehicle, there are two circuits inside the battery.
Firstly, lithium ions move from the cathode electrode to the anode electrode through the separator and electrolyte.
At the same time, the electrons of the cathode electrode move to the anode electrode through the wire, electrons and lithium ions react at the interface of the anode electrode material to generate lithium atoms which are then embedded in the graphite material.
Discharging
In the discharge process of lithium battery, there are also two circuits inside the battery.
Firstly, lithium ions move from the anode electrode to the cathode electrode through the separator and electrolyte.
Then, the electrons of the anode electrode move to the cathode electrode through the wire, electrons and lithium ions react at the interface of the cathode electrode material to generate lithium atoms which are then embedded in the electrode material.
“rocking chair batteries”
In this way, during the charging and discharging process, lithium ions move back and forth in the cathode and anode poles, like a rocking chair rocking around, so lithium-ion batteries are also called “rocking chair batteries”