Summarizing the materials of various lithium batteries, they can be divided into four categories:
- oxide
- carbon material
- alloy
- polymer.
The first category is oxides, including nickel-hydrogen battery cathode materials Ni(OH)2, lithium ion battery cathode materials LiCoO2, LiNiO2, LiNixCo1-xO2, LiMn2O4, V2O5, etc., as well as lithium ion battery oxide anode materials and solid oxide electrolytes, oxide catalytic electrodes of solid oxide fuel cells, etc.
The second category is carbon materials, including anode materials for lithium-ion batteries, such as graphite, hard carbon, and mesopause micro spheroidal carbon, etc.;
it also includes carbon nanotubes for supercapacitors, which can also be used as hydrogen storage materials, may become an important material in the field of hydrogen energy technology.
The third category is alloy, which mainly refers to the hydrogen storage alloy of the anode material of nickel-hydrogen battery and the tin-based alloy of the anode material of lithium ion battery, the latter is actually a lithium storage alloy.
The fourth category is polymers, including proton exchange membranes in proton exchange membrane fuel cells, polymer electrolytes in lithium-ion batteries, and polymer electrolytes in supercapacitors.
Lithium Ion Battery Materials
Lithium-ion battery materials
Lithium ion batteries are composed of the following components: cathode electrode, anode electrode, electrolyte, electrolyte salt, adhesive, separator, cathode electrode lead, anode electrode lead, center terminal, insulating material, safety valve, cathode temperature coefficient terminal (PTC terminal), anode current collector, cathode electrode current collector, conductive agent, battery case.
- The cathode material is lithium-containing transition metal oxides and phosphides such as LiCoO2, LiFePO4, etc., and conductive polymers such as polyacetylene, polyphenylene, polypyrene, polythiophene, active polysulfide compounds, etc.
- The anode material is carbon that can store a large amount of lithium basic materials, nitrides, silicon-based materials, tin-based materials, new alloys, etc.
- Electrolytes are organic solutions of lithium salts, polymers, and inorganic solids;
- Adhesives are fluoropolymers, ethylene-propylene rubber, carbon-based materials, and silica gel-based gels. glue, radioactive cross-linked polymer, etc.
- Separator material is porous polyolefin, polyamide non-woven fabric, etc.
- Cathode temperature coefficient terminal (PTC terminal) is a composite material of conductive filler and polymer.
- Anode current collector material is copper foil, copper mesh, stainless steel mesh, and other metal meshes.
- Cathode current collector material is aluminum foil, etc.
- Conductive agent is graphite, carbon black, acetylene black, colloidal carbon;
- Battery shell is made of steel, aluminum and other materials.
Cathode material
Lithium intercalation compound cathode material is an important part of lithium ion batteries.
The cathode electrode material occupies a large proportion in the lithium ion battery (the mass ratio of the positive and anode electrode materials is 3:1~4:1), so the performance of the cathode electrode material will greatly affect the performance of the battery, and its cost will directly determine if the battery cost is high and low.
At present, research on cathode materials mainly focuses on electrode materials such as lithium cobalt oxide and lithium nickel oxide.
At the same time, the rise of some new cathode materials (including conductive polymer cathode materials) has also injected new ideas into the development of lithium ion battery cathode materials.
Some battery manufacturers are seeking to develop a new system of lithium-ion battery cathode materials with high voltage, high specific capacity and good cycle performance is an important research content in this field.
LiFePO4 cathode material
LiFePO4 cathode material is a new type of cathode material for lithium ion batteries.
Due to the abundant iron resources, low price and non-toxicity, LiFePO4 is a positive electrode material for lithium-ion batteries with good development prospects.
LiFePO4 belongs to the olivine type structure, and the space group is Pnmb. In this structure, the voltage of Fe3+/Fe2+ relative to metal lithium is 3.4V, the theoretical specific capacity is 170mAh/g, and when LiFePO4 is oxidized to FePO¬4, the volume is reduced during the charging process, which can compensate for the expansion of the volume of the carbon anode electrode, thus to help improve the volume utilization of lithium-ion batteries.
However, the resistivity of LiFePO4 material is relatively large, and the utilization rate of electrode material is low, so the research work is mainly focused on solving the problem of its electrical conductivity.
Coating carbon and adding carbon to make composite materials is one of the effective way to improve the conductivity of LiFePO4 materials.
The capacity of the organic compound precursor by coating carbon before synthesis of LiFePO4 can reach 150 mAh/g, and after 10 cycles, there is only a 1% capacity loss.
Doping metal powders and organometallic salts is another way to improve the conductivity of LiFePO4 materials. S. Y. Chung et al. increased the conductivity of doped LiFePO4 by 8 orders of magnitude by doping a small amount of metal ions (Mg2+, Al3+, Ti4+, Zr4+, etc.).
LiFePO4 has high energy density, low price, and excellent safety, making it especially suitable for power batteries. Its appearance is a major breakthrough in lithium-ion battery materials, and it has become a hotspot for research in various countries.
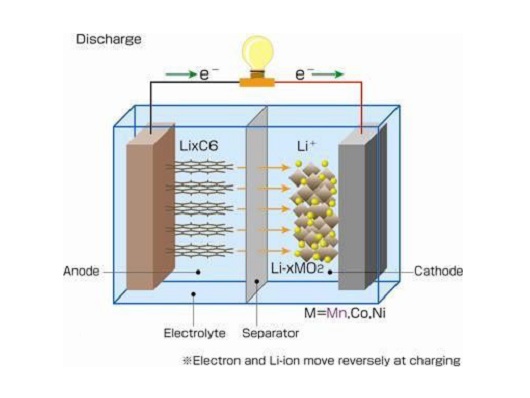
Anode material
The biggest difference between lithium ion batteries and secondary lithium batteries is that the former uses lithium intercalation compounds instead of metal lithium as the battery’s anode electrode.
Therefore, the research and development of lithium ion batteries is largely the research and development of anode lithium intercalation compounds.
As a negative electrode material for lithium-ion batteries, the necessary conditions are:
(1) Low electrochemical equivalent;
(2) The deintercalation of lithium ions is easy and highly reversible;
(3) The diffusion coefficient of Li+ is large;
(4) Good electronic conductivity;
(5) It is thermally stable and has good electrolyte compatibility, making it easy to make suitable electrodes.
At present, the anode electrode materials that have been actually used in lithium-ion batteries are basically carbon materials, such as artificial graphite, natural graphite, meso-phase carbon microspheres (MCMB), petroleum coke, carbon fiber, pyrolytic resin carbon, etc.
In addition, people are also actively research and develop non-carbon anode materials.
The current production of lithium-ion batteries has adopted a large number of graphite-like carbon materials as the anode electrode of the battery.
Graphite materials mainly include artificial graphite and natural graphite. Artificial graphite is made by graphitizing carbon (soft carbon) at high temperature.
Artificial graphite materials as anode materials for lithium-ion batteries mainly include graphitized microphase carbon microspheres, graphite fibers and various other graphitized carbons. Among them, the most familiar is the highly graphitized microphase carbon microspheres (HGMCMB).